
Mass (amu) Abundance Isotope 46TI 48TI 45.95263 76.300 15.000 47.94795 50TI 49.94479 8.700 What is the average atomic mass of titanium on that planet average atomic mass. On another planet, the isotopes of titanium have the given natural abundances.
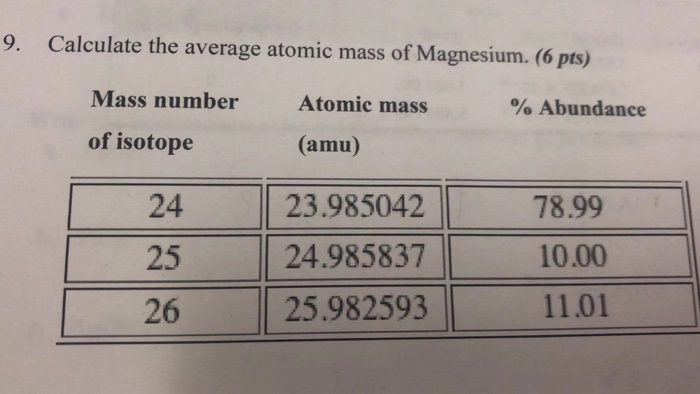
The average atomic mass of titanium will thus beĪvg. hexagonal close-packed (hcp) Titanium is a chemical element with the symbol Ti and atomic number 22. average atomic mass ( abundance of each isotope / 100. The decimal abundances for these five isotopes will be: Simply put, each isotope i will contribute to the average atomic mass of the element in proportion to its decimal abundance, which is simply the percent abundance divided by 100.Ī v e r a g e a t o m i c m a s s = ∑ i m a i × a b u n d a n c e o f i Isotope Abundance Mass (u) 46Ti 77.800 45.95263 48Ti 14.400 47.94795 50Ti 7.800 49.94479 What is the average atomic mass of titanium on that planet On another planet, the isotopes of titanium have the given natural abundances. calculate the average atomic mass of oxygen on earth. Now, the average atomic mass of titanium is calculated by taking the weighted average of the atomic masses of its stable isotopes. What is the average atomic mass of titanium if we have an isotopic distribution of 78.5 of ''46Ti, 45.95263'amu', 12.3 of ''48Ti, 47.94795'amu', and. titanium 60.10 of a different sample of titanium dioxide is titanium. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.When the problem doesn't provide you with the actual atomic mass of an isotope, m a , you can use its mass number, A, as an approximation of its atomic mass. Please note that the elements do not show their natural relation towards each other as in the Periodic system. The unity for atomic mass is gram per mol. On another planet, the isotopes of titanium have the given natural abundances.
The lightest chemical element is Hydrogen and the heaviest is Hassium. Calculate the average atomic mass of bromine based on these experiments. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu, respectively) and abundances (50.69 and 49.31, respectively) were determined in earlier experiments. Isotope Abundance Mass (u) 46 Ti 76.000 45.95263 48 Ti 15.600 47.94795 50Ti 8.400 49. Average atomic masses listed by IUPAC are based on a study of experimental results. The chemical elements ofįor chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). On another planet, the isotopes of titanium have the given natural abundances. This list contains the 118 elements of chemistry. Separation and Concentration Purification RequestĬhemical elements listed by atomic mass The elements of the periodic table sorted by atomic massĬlick on any element's name for further information on chemical properties, environmental data or health effects.Plant Inspection & Process Optimalisation Isotope Abundance Mass (u) 46Ti 71.800 45.95263 48Ti 11.900 47.94795 50Ti 16.300 49.94479 What is the average atomic mass of titanium on that planet On another planet, the isotopes of titanium have the given natural abundances.


 0 kommentar(er)
0 kommentar(er)
